Neutron Activation Analysis
Introduction
Instrumental Neutron Activation Analysis (INAA) is one of the most sensitive analytical techniques used for the quantitative multi-element analysis of major, minor, and trace elements in samples from almost every conceivable field of scientific or technical interest. The technique of INAA measures the total amount of an element present in sample matrices without regard to chemical or physical form and without any pre-treatment of the sample. For certain elements, INAA offers sensitivities that are superior to those possible by other techniques; on the order of parts per billion or better. In addition to the elemental and isotopic analysis of samples, INAA permits the analysis of non-radioactive tracers introduced into biological, chemical, and/or industrial processes for process identification and optimization.
Applications of INAA
Samples analyzed via INAA at NC State typically fall into one of the four general categories listed below. For informational purposes, sample plots including detection limit (MDL) data have been attached for select elements in certain sample matrices.
I. Agricultural, Biological and Environmental Samples:
Agricultural products, benthic organisms, biological tissues & fluids (blood, hair, nails, organs, urine), bone, fertilizer, fish (tissue and eggs), food products, forensic samples, plants, sediment, sludges, vegetation, water, wood.

Examples of elemental analyses performed on agricultural, biological and environmental samples at N.C. State include:
- Antimony in Fertilizer
- Arsenic in Fish Tissue
- Arsenic in Human Nails
- Chromium in Fertilizer
- Cu in Benthos
- Copper in Primate Liver
- Gold in Rat Muscle
- Magnesium in Sludge Waste
- Manganese in Rat Brain Tissue
- Mercury in Fish Tissue
- Mercury in Soil
- Rubidium in Food
- Selenium in Fish Tissue
- Uranium in Water
- Tungsten in Water
- Zinc in Primate Liver Tissue
II. Industrial Samples: Crude oil, filter media & resins, fly ash, plastics, synthetic fibers, textiles.

Examples of elemental analyses performed on industrial samples at N.C. State include:
- Palladium in Polymers
- Samarium in Textiles
- Selenium in Crude Oil
- Tin in Polyester Resin
III. High Purity Matrix Samples: Carbon/graphite, ceramics, chemicals, metallic alloys, pharmaceuticals, refractory kiln bricks, semiconductor substrates and processing materials.

Examples of elemental analyses performed on high purity matrices at N.C. State:
- Sodium in Fused Silica
- Chlorine in Organic Polymers
IV: Geologic Samples: Coal, ores, rocks, soils/sediments.

Examples of elemental analyses performed on geologic samples at N.C. State:
- Arsenic in Sediment
- Chromium in Soil
- Iridium in Geologic Samples
Advantages of using INAA for trace element analysis:
- It is a multi-element technique capable of determining approximately 65 elements in many types of materials (see list of elements below*)
- It is non-destructive and therefore, does not suffer from the errors associated with yield determinations;
- It has very high sensitivities for most of the elements that can be determined by INAA – most detection limits range from ~0.05 to ~50 ppm (≤ 1 ppb for some high-purity materials)
- It is highly precise and accurate;
- It permits the analysis of samples ranging in volume from 0.1 ml to 20 ml, and in mass from ~0.001 gram to 10 grams depending on sample density.
- Samples for INAA can be solids, liquids, gases, mixtures, and suspensions.
| *Elements that may be analyzed via INAA include: Ag, Al, As, Au, Ba, Br, Ca, Cd, Ce, Cl, Co, Cr, Cs, Cu, Dy, Er, Eu, F, Fe, Ga, Gd, Ge, Hf, Hg, Ho, I, In, Ir, K, La, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, Os, Pd, Pr, Pt, Rb, Re, Rh, Ru, Sb, Sc, Se, Si, Sm, Sn, Sr, Ta, Tb, Te, Th, Ti, Tm, U, V, W, Y, Yb, Zn, & Zr. |
Neutron Irradiation & Activation:
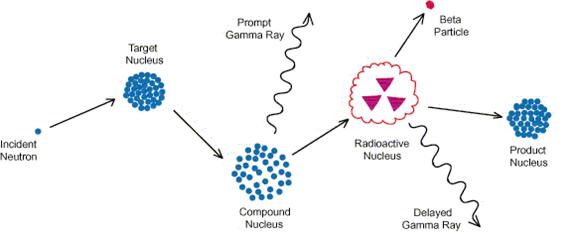
The NAA laboratory at North Carolina State University utilizes the 1-MW PULSTAR Nuclear Reactor facility as an intense neutron source for the irradiation of client samples. During neutron irradiation, certain stable isotopes of elements that constitute the samples are transformed into radioactive isotopes by neutron capture (see Fig #1 above).
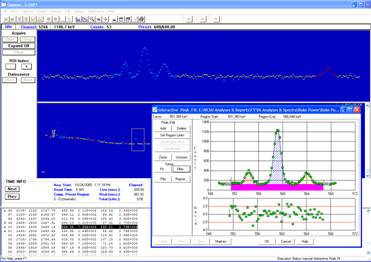
Activated trace ‘radioisotopes’ (e.g. As-76, Hg-197, Se-75, etc…) contained in the samples are analyzed and quantified by utilizing gamma spectrometry systems (see Fig#2) to measure gamma ray decay signatures. Nominal sensitivities for select elemental analysis are given under the applications section above.
Certified elemental standards, traceable standard reference material (SRM) controls, method blanks, and sample duplicates are processed along with client samples to maintain a high degree of quality assurance.

For additional information about NAA, please contact the Manager of Nuclear Services.